[ad_1]
Plants range from simple seaweeds and single-celled pond scum, through to mosses, ferns and huge trees. Palaeontologists like us have long debated exactly how this diverse range of shapes and sizes emerged, and whether plants emerged from algae into multicellular and three-dimensional forms in a gradual flowering or one big bang.
To answer this question, scientists turned to the fossil record. From those best-preserved examples, like trilobites, ammonites and sea urchins, they have invariably concluded that a group’s range of biological designs is achieved during the earliest periods in its evolutionary history. In turn, this has led to hypotheses that evolutionary lineages have a higher capacity for innovation early on and, after this first phase of exuberance, they stick with what they know. This even applies to us: all the different placental mammals evolved from a common ancestor surprisingly quickly. Is the same true of the plant kingdom?
In our new study, we sought to answer this question by looking for certain traits in each major plant group. These traits ranged from the fundamental characteristics of plants – the presence of roots, leaves or flowers – to fine details that describe the variation and ornamentation of each pollen grain. In total, we collected data on 548 traits from more than 400 living and fossil plants, amounting to more than 130,000 individual observations.
We then analysed all this data, grouping plants based on their overall similarities and differences, all plotted within what can be thought of as a “design space”. Since we know the evolutionary relationships between the species, we can also predict the traits of their extinct shared ancestors and include these hypothetical ancestors within the design space, too.
For example, we will never find fossils of the ancestral flowering plant, but we know from its closest living descendants that it was bisexual, radially symmetric, with more than five spirally arranged carpels (the ovule-bearing female reproductive part of a flower). Together, data points from living species, fossils and predicted ancestors reveal how plant life has navigated design space through evolutionary history and over geological time.
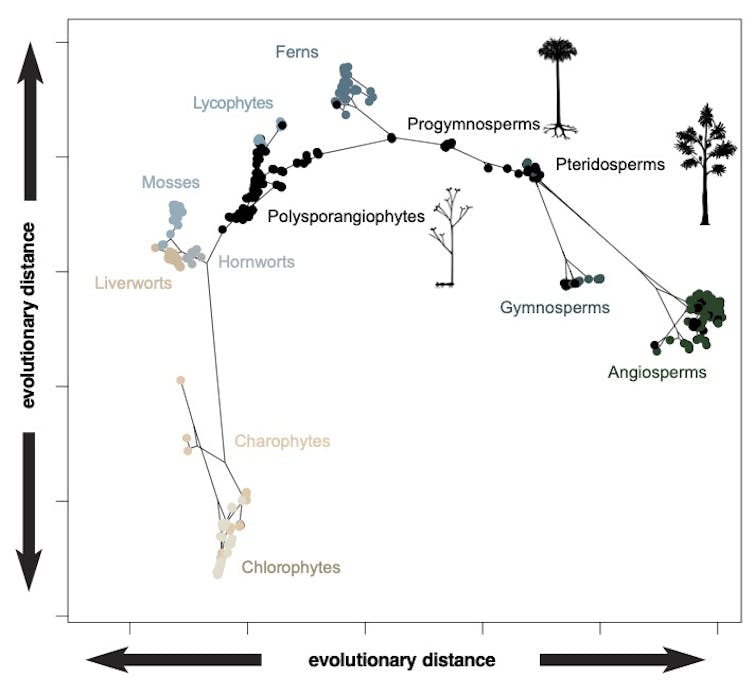
Philip Donoghue et al / Nature Plants
We expected flowering plants to dominate the design space since they make up more than 80% of plant species, but they don’t. In fact, the living bryophytes – mosses, liverworts and hornworts – achieve almost as much variety in their body forms.
This may not be entirely surprising since the three lineages of bryophytes have been doing their own thing for more than three times as long as flowering plants. And despite their diminutive nature, even the humble mosses are extraordinarily complex and diverse when viewed through a microscope.
The evolutionary relationships conveyed by the branching genealogy in the above plot show that there is, generally, a structure to the occupation of design space – as new groups have emerged, they have expanded into new regions. However, there is some evidence for convergence, too, with some groups like the living gymnosperms (conifers and allies) and flowering plants plotting closer together than they do to their common ancestor.
Nevertheless, some of the distinctiveness of the different groupings in design space is clearly the result of extinction. This is clear if we consider the distribution of the fossil species (black dots in the above figure) that often occur between the clusters of living species (coloured dots in the figure).
So how did plant body plan diversity evolve?
Overall, the broad pattern is one of progressive exploration of new designs as a result of innovations that are usually associated with reproduction, like the embryo, spore, seed and flower. These represent the evolutionary solutions to the environmental challenges faced by plants in their progressive occupation of increasingly dry and challenging niches on the land surface. For example, the innovation of seeds allowed the plants that bear them to reproduce even in the absence of water.

ANGHI / shutterstock
Over geological time, these expansions occur as episodic pulses, associated with the emergence of these reproductive innovations. The drivers of plant anatomical evolution appear to be a combination of genomic potential and environmental opportunity.
Plant disparity suggests that the big bang is a bust
None of this fits with the expectation that evolutionary lineages start out innovative before becoming exhausted. Instead, it seems fundamental forms of plants have emerged hierarchically through evolutionary history, elaborating on the anatomical chassis inherited from their ancestors. They have not lost their capacity for innovation over the billion or more years of their evolutionary longevity.
So does that make plants different from animals, studies of which are the basis for the expectation of early evolutionary innovation and exhaustion? Not at all. Comparable studies that we have done on animals and fungi show that, when you study these multicellular kingdoms in their entirety, they all exhibit a pattern of episodically increasing anatomically variety. Individual lineages may soon exhaust themselves but, overall, the kingdoms keep on innovating.
This suggests a general pattern for evolutionary innovation in multicellular kingdoms and also that animals, fungi and plants still have plenty of evolutionary juice in their tanks. Let’s hope we’re still around to see what innovation arises next.
[ad_2]
Source link




















